Drugging the RNA: part I, Why do it?
In part I of a series of posts discussing how to drug RNA, we discuss the three dimensions on which a disease target can be drugged, and how RNA’s dual role as both blueprint and object confers it unique properties that make it a particularly attractive target dimension over protein and DNA.
In the drug discovery space, the lion’s share of attention is often directed at the myriad of ways to drug a disease target, which assumes that the correct target had been chosen in the first place. As we’ve written about previously, in the vast majority of cases, this is a false assumption as exemplified by the high failure rate of trialed drugs. At Genetic Leap, we built our Genetic Intelligence capabilities to specifically address the hard challenge of target selection. This is because without the correct target, even the most ingenious drug design approach is doomed from the outset. Once a suitable target has been selected, drugging said target becomes the next challenge.
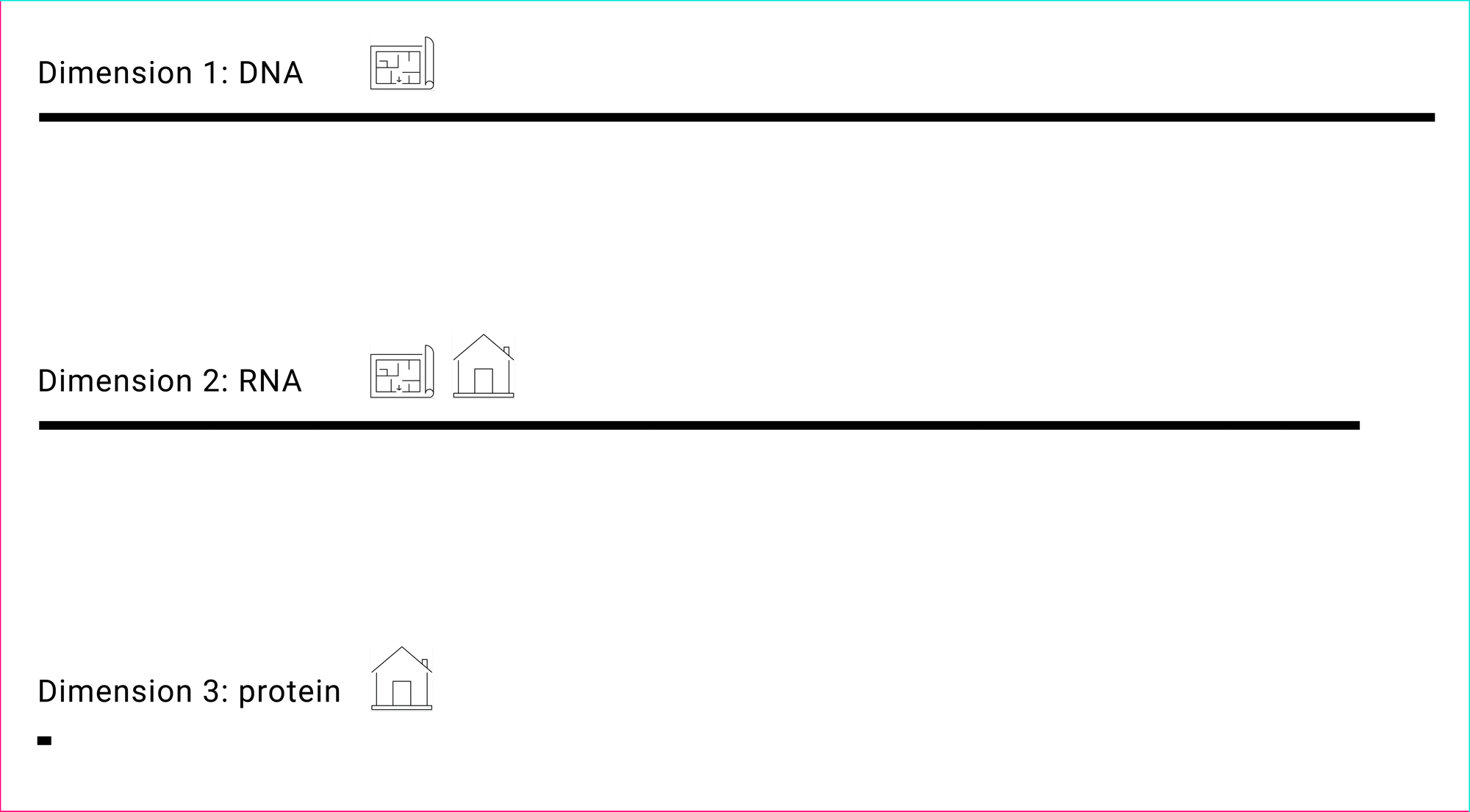
A target has three dimensions at which an intervention can be applied to modulate it: dimension 1 (DNA), dimension 2 (RNA) and dimension 3 (proteins). These dimensions are derived from the central dogma of molecular biology, a description for how genetic information flows from blueprints to objects/devices to drive biological life. Dimension 1 serves as a blueprint. Dimension 2 is popularly known to serve as a blueprint, but it also serves a major role as objects. And finally, dimension 3 serves as objects.
Dimension 3: proteins
Traditionally, most drugs have taken aim at dimension 3 using small molecules. The advantage of intervening at dimension 3 is that it is the dimension that we understand the most and have a vast, hard-earned trove of knowledge on in terms of do’s and don’ts on how best to drug it. Most drug discovery experts understand this dimension the most, and universities primarily train researchers and medical practitioners from the perspective of addressing this dimension. The entire system is geared at this dimension.
Furthermore, the primary device of intervention on dimension 3 is small molecules, and they are easy to make, stable at room temperature for a long time, and easy to administer (often orally available).
Lastly, changes made to dimension 3 are typically reversible, meaning that if a patient stops taking the drug, the target can return to what it was prior to the intervention. This is a good attribute to have from a safety perspective (though maybe not from a convenience standpoint).
One of the key downsides of dimension 3 is that proteins only account for about 1% of the genome, which means that most causal disease targets do not have dimension 3 but rather only have dimension 1 and 2. Making matters worse, the vast majority of proteins are undruggable, meaning that they don’t have a deep enough pocket for a small molecule to bind to. Only about 400 proteins have been drugged (out of an estimated 100,000 to 400,000 in the human body), leaving the vast majority of proteins as undruggable. It’s worth nothing that there are countless efforts to drug undruggable proteins (e.g., using PROTACs, covalent inhibitors, and other strategies), but these workarounds have special requirements and are not generally applicable. Furthermore, intervention on the proteins dimension is often limited to gain of function situations (where the target should be downregulated), leaving loss of function situations often without recourse.
Peptide-based modalities provide another strong option to intervene on dimension 3, but barring some that fit a specific profile (e.g., monoclonal antibodies), they are often limited by immunogenicity problems, not to mention issues with manufacturing, stability, and administration. Often times, this is limited to gain of function targets, i.e., targets that should be downregulated.
Dimension 1: DNA
The dream of precision medicine was kindled with the elucidation of the DNA code and has since been popularly tied to intervention directly at the DNA using gene therapy. Recent advances like CRISPR made that dream seemingly all the more attainable.
The advantage of intervention at dimension 1 would be a one-time repair, and the problem is permanently fixed at the source. Furthermore, reaching to dimension 1 can address gain of function (need downregulation) as well as loss of function (need upregulation) targets. More still, every potential disease targets is reachable compared to a vanishing proportion when targeting dimension 3.
The reality is of course far more complicated than the advertised dream, with numerous problems that will likely keep that dream at bay for the foreseeable future. For one, gene therapy often has immunogenicity problems, typically stemming from the delivery vehicle which is itself often viral. Advances are being made with non-viral delivery vehicles, but these aren’t as efficient as the viral counterparts.
Another problem is that the intervention device are often too big to fit inside the delivery vehicles, though research efforts are underway to find smaller and smaller ones.
Yet another problem is that the intervention can cause off target changes (mutations) to unintended regions of dimension 1, leading to mosaicism and other complications. There are suggested mitigations (e.g., performing treatment on cells outside the body, filtering these cells for no off-target changes, and re-implanting cells with the correct changes). However, such mitigations would come with additional complexity, time delays and costly expense.
Then there’s also the reality that—barring cases were non-integrative supplementation is the strategy and making no mention of immunogenicity compromise—genetic changes made to somatic cells will not propagate as they are not germ-line. On the surface, the intervention might be of limited therapeutic use beyond zygote stage. Making the genetic changes on stem cells and re-introducing those stems cells into the patient are some of the solutions that will hopefully be fruitful.
Dimension 2: RNA
And then there’s RNA (dimension 2). Because it plays an extensive dual role as both blueprint and object, RNA has all of the properties that make DNA (dimension 1) and protein (dimension 3) attractive as ground for intervention.
As with DNA, intervention at the RNA comes with the versatility and flexibility of targeting the source blueprint. Both gain of function and loss of function situations can be addressed. Moreover, the vast majority of possible targets are accessible, which constitute an ultra expansion of the target space compared to dimension 3 (proteins). With the ability to intervene at dimension 2, no target is undruggable or unreachable.
And yet, as with proteins on dimension 3, especially if using small molecules as the intervention device, the drugs can be convenient, easily administered (e.g., orally), devoid of immunogenicity complications, and reversible.
So drugging the RNA sounds incredible. It’s genetic control without the downsides of DNA-based gene therapy. But what’s the catch?
The catch is emphatic. Drugging the RNA requires solving some seriously big challenges that scientists have been hard at work trying to address for decades. We’ll explore some of these considerations in subsequent posts, namely:
- In part II: the two strategic approaches to drugging the RNA
- In part III: the two technical approaches to drugging the RNA
- In part IV: the three stages to drugging a specific RNA

